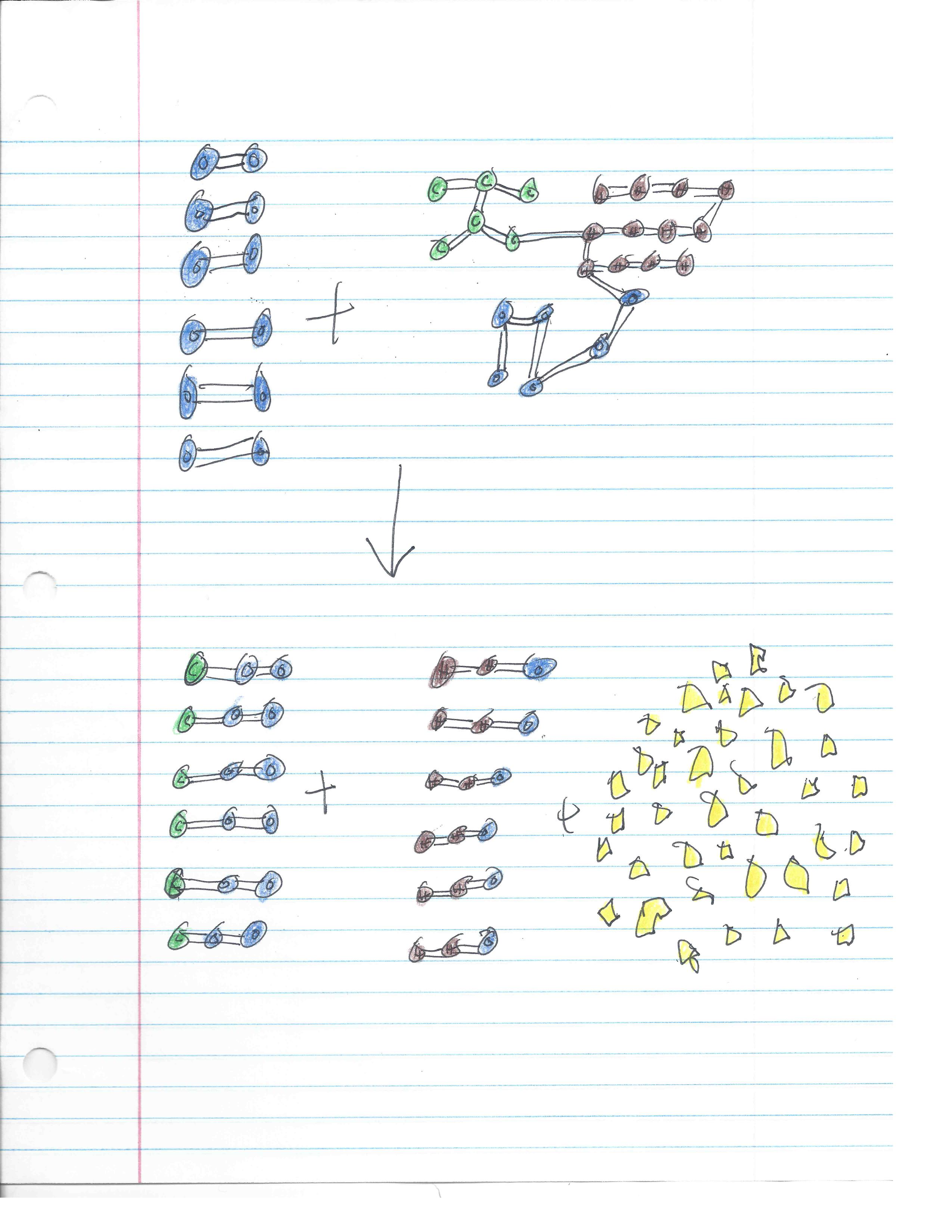
More Below
↓
Blue=O Molecules
Green=C Molecules
Brown=H Molecules
Yellow=Energy Molecules
6 O2 + C6H12O6 → 6 CO2 + 6 H2O + 38 Energy Molecules
What is happening to these 6 O2 molecules and one C6H12O6 molecule, otherwise known as 6 oxygen molecules and 1 gluctose molecule, is simple. There is a chemical reaction between the 7 molecules and they are transformed into something completely different. They are transformed into 6 CO2 and 6 H2O and 38 Energy Molecules. This proves the law of conservation of energy because there are the same amount of each molecule, such as O, C, and H molecules, on both sides. The law of conservation of energy states that all energy is always there, or that energy cannot be created or destroyed. Cellular respiration is when a plant or animal takes glucose and oxygen and changes it into carbon dioxide, water, and energy.